Rufen Sie uns einfach an, und wir beraten Sie gerne zu unserem Seminar- und Studienangebot.
Unsere Ansprechpartner:

Michael Rabbat, Dipl.-Kfm.
MBA Chief Operating Officer

Claudia Hardmeier
Kunden-Center
Studienbetreuung
5.2.2. Critical Quality Attributes for Product Score Cards
Test criteria defining quality
|
Impacting on
|
(determined by physical and chemical stability of drug product during storage at either room temperature or accelerated conditions)
Then risk mapping was used to identify materials and processes together with their critical material attributes and critical process parameters impacting these product scores.
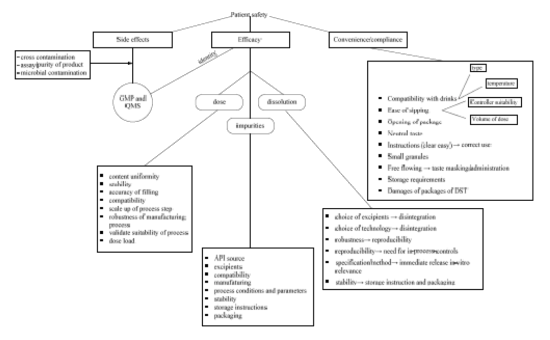
Figure 9: Risk mapping for ClaroSip ®63
63 Source:author







