Rufen Sie uns einfach an, und wir beraten Sie gerne zu unserem Seminar- und Studienangebot.
Unsere Ansprechpartner:

Michael Rabbat, Dipl.-Kfm.
MBA Chief Operating Officer

Claudia Hardmeier
Kunden-Center
Studienbetreuung
2.1. International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines aiming to improve the quality of drug products
In combination the guidelines ICH Q8(R2), ICH Q9 and ICH Q10 introduce a shift of paradigms in how to develop drug products in the pharmaceutical industry. This means breaking with the traditional approach that defined quality mainly as CGMP compliance during manufacturing and testing of drug products under the surveillance of a QA system in conformance with specified acceptance criteria, as approved in the marketing authorisation of the drug product.
The new paradigm emphasizes a different perspective of quality and how it can be ensured consistently throughout a product’s lifecycle:29
- Quality must be mainly built in and it will not only improve by additional testing and inspection
- Better utilization of modern science throughout product lifecycle
- QRM is a key enabler throughout product lifecycle
- Robust PQS, with appropriate knowledge management, assures quality throughout product lifecycle
- An integrated approach to development, manufacturing and quality for both industry and regulators
ICH Q8(R2) describes the elements of the pharmaceutical development part of the drug product in the dossier and all contents that are required in this section for filing an application for marketing authorisation. Furthermore the guideline defines and explains how to use and apply the elements of QbD to the pharmaceutical development and how and where to include these in the CTD. 30 ICH Q9 describes the overall risk management process of the Quality Risk Management (QRM) and its main steps of risk assessment, risk control, risk communication and risk review. It explains how quality risk management should be integrated into industry and regulatory operations as part of a company’s pharmaceutical quality system and summarises the methodology of risk management to support a scientific and practical approach to decision-making. It also cites methods to accomplish the quality risk management process based on current knowledge assessing the probability, severity or criticality and detectability of each risk.31
ICH Q10 aims to complement regional CGMP requirements, ISO standards and ICHQ7 to achieve product realisation, maintain a state of control and facilitate continual improvement (throughout the lifecycle from pharmaceutical development, technology transfer, commercial manufacturing to product discontinuation). It suggests a quality manual emphasising overall management responsibility to ensure an effective pharmaceutical quality system is in place to achieve the quality objectives. The roles, responsibilities, and authorities need to be defined, communicated and implemented throughout the company. A PQS also covers the management of outsourced activities and purchased materials and the management of change in product ownership. Knowledge Management and Quality Risk Management are the enablers for an effective and successful implementation of ICHQ10. Continual Improvement of process performance and product quality is then achieved through the implementation of these PQS elements.32
The following three figures outline the key elements of a QbD driven development and how the three aligned ICH guidelines should be used throughout all phases of drug development, starting at the definition of the Target Product Profile, throughout drug product development, process development, scale-up of processes, and continuing through transfer into commercial scale manufacturing and process validation - thus carrying on throughout the complete lifecycle of a product until its final discontinuation. The main shift of paradigms as pursued by a QbD driven development could therefore be summarised as a customer driven approach focussing on criticality and science as the foundations of all development activities. These are enabled by continuous risk assessment, knowledge management and continual improvement, combined into a reciprocating cycle throughout a product’s lifetime.

Figure 2: QbD flow chart from ICH Q-IWG Integrated Training Programme of ICH Quality Implementation Working Group33
development activities" class="wp-image-10004 size-full" height="437" src="https://sgbs.ch/wp-content/uploads/Figure-3-Linking-quality-risk-management-to-other-QbD-elements-and-development-activities.png" width="576"> Figure 3: Linking quality risk management to other QbD elements and development activities34
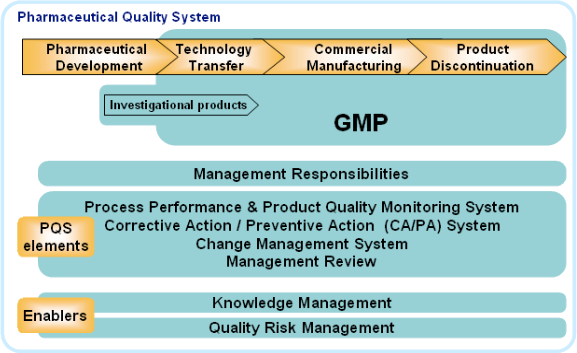
Figure 4: Own adaptation of pharmaceutical quality system model from ICH Q10l35
29 Robert; ICH Q-IWG Integrated Training Programme; slide 5
30 ICH Q8(R2); 2009
31 ICH Q9; 2006
32 ICH Q10; 2009
33 Source: ICH Q-IWG Integrated Training Programme; 2010; slide 7 (slightly adapted)
34 Source: Nasr ; Implementation of Quality by Design (QbD); 2011
35 Source: ICH Q10; 2009 (slightly adapted)







