Rufen Sie uns einfach an, und wir beraten Sie gerne zu unserem Seminar- und Studienangebot.
Unsere Ansprechpartner:

Michael Rabbat, Dipl.-Kfm.
MBA Chief Operating Officer

Claudia Hardmeier
Kunden-Center
Studienbetreuung
4.1. Generating core customer value via product quality and price
The product as part of the four Ps of the marketing mix is the most direct one to generate the core customer value and is build of two parts. The actual product is differentiated by its brand name, quality level, packaging, design and features, and the augmented product comprising delivery, product support, warranty and after-sale service.50 As such product quality can be distinguished either as the excellence it provides in its features, design, packaging or handling for the customer or the reliability at which the proposed value proposition for the customer can be provided.51
While the prescription drug market strictly speaking is a business to business market as drug products are sold to pharmacies, physicians and hospitals, who are in most cases making the actual buying decisions (even though they are not necessarily delivered to or paid for by these customers).The final customer of drugs being the patient, will be most directly affected by the product’s value proposition, by product innovation, and by the product quality.
The regulatory authorities of each country have been given the mandate to protect public health and patients from inefficacious and unsafe drug products and do so by evaluating the applications from pharmaceutical companies for marketing authorisation of their new products (NDA or MAA), based on the data provided in the submission documents on the efficacy, safety and quality of each drug product and by performing routine inspections of the manufacturing facilities. They thus represent a powerful stakeholder or gatekeeper for the product’s entry to the market and continuation on the market.
A further complication in this customer relationship exists in the common practice that in most countries drugs are actually paid for neither by the customers making the buying decision, nor the patients themselves but either by a public health care system or by health care insurance providers, that either directly pay or reimburse the drug expenditures of their members. As these represent powerful buyers deciding on which drugs to ay for or not they also do have significant impact on a pharmaceutical company’s freedom on pricing options as discussed in Chapter 1.1.
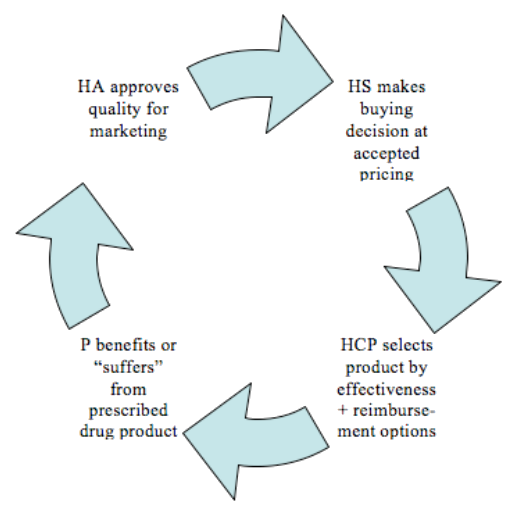
Figure 5: Schematic drawing of the buying transaction cycle for prescription drug products; P = patient. HA = national health authority, HS = health care system, HCP = health care provider (physician, hospital,..)52
Therefore the perceived value proposition of the product, i.e. the drug compound in its specific application form, its dosage form and its packaging will be different for each of these different customers, based on their role in the buying transaction and their use of the product.
National health authorities: seek excellence of marketed drug products for the sake of patients as to efficacy and safety and require high quality of conformance and consistency
Health care systems: seek maximisation of the perceived value at the lowest possible price position for a specific product as compared to alternative treatment options (the same for more or less for more pricing)
Physicians, hospitals, (pharmacies),..: seek efficacious and safe treatment for their patients at reduced resources to do so; thereby reducing their own costs for the provided service or increasing their efficiency in doing so.
Patients: seek product excellence, i.e. efficacious, safe drugs that are convenient, easy to use, and allow them to maximise quality of life
While authorities and patients are mostly concerned about the quality as to efficacy, safety, design, features and handling of the product, health care providers and health care systems are much more interested in the relative quality of these products compared to the pricing position and thus the cost efficiency of the drug product.
While QbD was rolled out by the authorities to increase their value proposition of new drugs and to foster increased value for patients, the different needs and expectations for the value proposition of a drug product from the other members of this buyer circle also need to be considered when evaluating the competitive advantage QbD can generate via superior product quality.
A. Selen from the FDA summarised the desired quality with focus on the patient at a conference as53
- “Product: designed to meet intended use and consistently delivers the desired/intended dose”
- “Manufacturing Process: designed to consistently meet product critical quality attributes and suitable for continuous monitoring and updates, and allows for consistent quality over time (life cycle)”
- “Understanding the Main Sources of Variability: due to starting materials and process, due to methodology and assumptions, due to product-patient interface and the patient
50 Kotler; Principles of Marketing; p 250: Three levels of product
51 Jones; Theory of Strategic Management; p 86-87: quality as excellence and reliability
52 Source: author
53 Selen; QbD and IVIVC/R: Ensuring Product Quality and Patient Benefit; 2008; slide 6







