Rufen Sie uns einfach an, und wir beraten Sie gerne zu unserem Seminar- und Studienangebot.
Unsere Ansprechpartner:

Michael Rabbat, Dipl.-Kfm.
MBA Chief Operating Officer

Claudia Hardmeier
Kunden-Center
Studienbetreuung
8.3. The future focus of the FDA to promote QbD
The actions taken after that report had obviously not been able to generate as much positive impact as hoped for and this was also obvious from the summary findings on the recent experiences and remaining hurdles to the implementation of QbD. The presentation during a FDA meeting in July 2011 highlighted again similar deficiencies at the regulatory bodies and the continuing lack of industry’s acceptance due to remaining concerns and lacking integration into the corporate structure and systems.
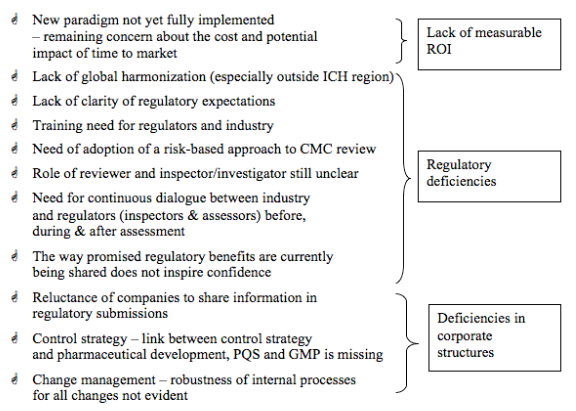
Figure 15: Persistent obstacles to widespread implementation of the new paradigm of QbD: current perspectives on opportunities and challenges78
An additional approach to support the adoption of QbD also targets to a different direction now, trying to promote new manufacturing technologies, which are more amendable to the systematic development and monitoring approach, and to promote new PAT technologies and statistical tools as summarised in the FDA’s strategic plan in August 2011.79
78 Source: Nasr; Implementation of Quality by Design (QbD); 2011; slide 31-32 (slightly adapted)
79 FDA; Advancing Regulatory Science at FDA A Strategic Plan August; 2011







